
Method validation is the process used to confirm that the analytical procedure employed for a specific test is suitable for its intended use. Results from method validation can be used to judge the quality, reliability and consistency of analytical results; it is an integral part of any good analytical practice. Analytical methods need to be validated or revalidated
- before their introduction into routine use;
- whenever the conditions change for which the method has been validated (e.g., an instrument with different characteristics or samples with a different matrix); and
- whenever the method is changed and the change is outside the original scope of the method.
The validity of a specific method should be demonstrated in laboratory experiments using samples or standards that are similar to unknown samples analyzed routinely. The preparation and execution should follow a validation protocol, preferably written in a step-by-step instruction format. Possible steps for a complete method validation are listed in Table 1. This proposed procedure assumes that the instrument has been selected and the method has been developed. It meets criteria such as ease of use; ability to be automated and to be controlled by computer systems; costs per analysis; sample throughput; turnaround time; and environmental, health and safety requirements.
ISO/IEC 17025 definition: ‘The confirmationby examination and the provision of objective evidencethat the particular requirements for a specific intended use are fulfilled’
What is validation?
- Specific intended use
– analytical requirement
– what will test method be used for? why are measurements required?
- Objective evidence
– experimental data from method validation study
– information on method performance
- Confirmation
– comparison of requirement with experimental evidence
Can the method deliver results that are fit for a particular purpose?
Why is method validation necessary?
- Good science
- Essential for ensuring reliability of results
- Consistent application of methods
- Better agreement between analysts/laboratories/ countries
- Key requirement of accreditation standards–ISO/IEC 17025
Without validation there can be no assurance that results will be fit for purpose
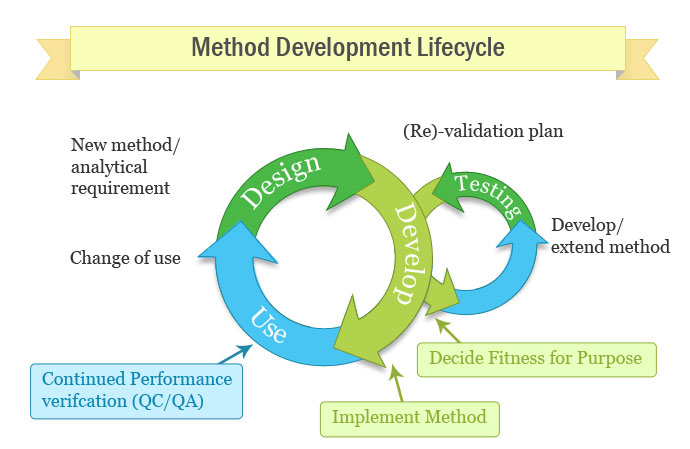
When do you validate a method?
- During method development
– is the proposed method likely to be fit for purpose?
- Before using any method for analysis of test samples
– including standard/published methods which have been validated by others
– verify suitability for analytical requirement
– verify own ability to match published data
- Change of application/working environment/analyst
- Following period of non-use
ISO/IEC 17025 Selection of methods and method validation
- §5.4.1 General
- laboratory shall use appropriate methods and procedures for all tests within its scope
- §5.4.2 Selection of methods
- methods must meet customer requirements
– preference for standard methods (international/national standards)
– ‘laboratory shall confirm that it can properly operate standard methods before introducing the tests’
– laboratory-developed methods may be used if they are validated
§5.4.5 Validation of methods
- ISO definition
- Types of method that require validation
– (standard -§5.4.2)
– non-standard
– laboratory designed/developed
– standard methods outside normal scope
– amplified/modified standard methods
- Validation required to demonstrate that methods are fit for their intended use
Extent of validation
- As extensive as necessary for application
- Must record validation data and document procedures used
- Include statement of ‘fitness for purpose’
- Method performance must be relevant to customer needs and assessed against intended use of the method
- measurement uncertainty, detection limit, selectivity, linearity, repeatability/reproducibility, robustness
Defining the analytical requirement
- Why are the measurements required?
– what will the data be used for?
- Analyte
– quantitative or qualitative
– class/total/available/species
– likely concentration
- Sample
– nature (matrix)
– size
- Other considerations
– destructive vs non-destructive test
– timescale
– cost
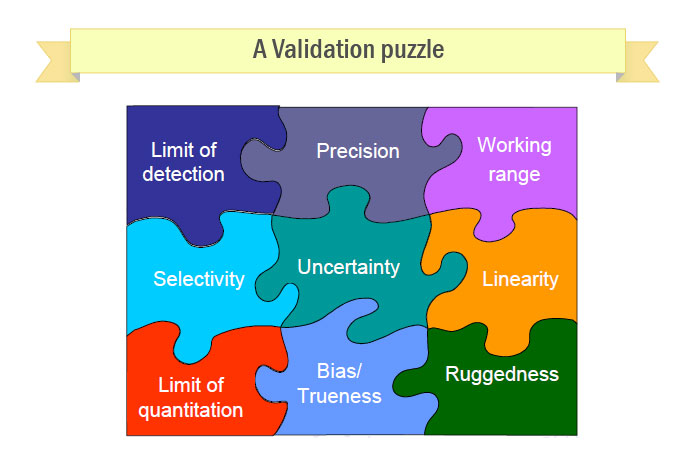
Method performance parameters
- Selectivity/specificity
– am I measuring what I think I’m measuring? are there any interferences?
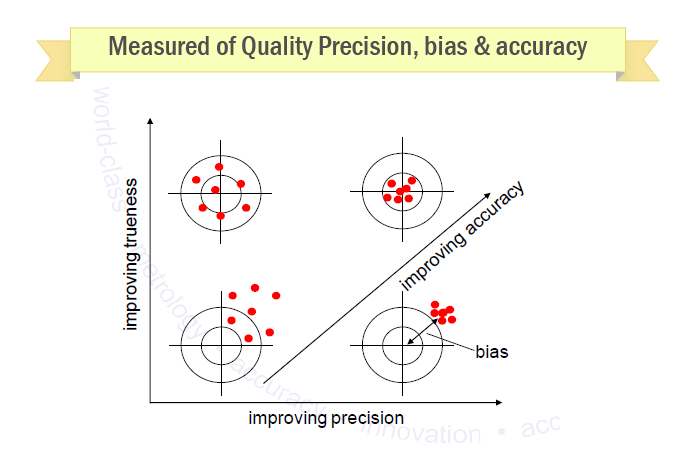
- Precision (repeatability, reproducibility)
– how close are the results of replicate measurements made on the same sample?
- Bias, recovery
– how close are the results to the ‘right’answer
- Working range
– range of analyte concentrations that can be measured reliably
– limit of detection, limit of quantitation
- Ruggedness/robustness
– control necessary for each stage of the procedure
How Much Validation?
Depends on the criticality of the measurement, scope of the method, level of information and experience already available
| Parameter | Type of Analysis | |||
|---|---|---|---|---|
| Qualitative | Major Component | Trace Analysis | Physical Property | |
| Specficity / selectivity |  |
 |
 |
 |
| Precision |  |
 |
 |
|
| Bias |  |
 |
 |
|
| Limit of Detection |  |
 |
||
| Limit of Quantitation |  |
|||
| Linearity/working range |  |
 |
 |
|
| Ruggedness |  |
 |
 |
 |
Major component : analyte concentration in range 1-100%
Trace analysis : analyte concentration less than 100 mg/kg
Fitness for purpose
- Analyse data from method performance parameters
- Are target values achieved?
– YES - method is fit for purpose
– NO - more development required
- Method is validated by the declaration of fitness for purpose
Summary
- Method validation is required to produce meaningful data
- Both in-house and standard methods require validation/verification
- Key requirement of ISO/IEC 17025
- Validation should be a planned activity
– parameters required will vary with application - Validation is not complete without a statement of fitness for purpose
Send Enquiry !
Our Services
Our Clientele
The Logos are the property of the respective companies. There are use here just for the representation sake















