A Recognition of Testing Competence

Testing laboratories seeking ISO/IEC 17025 will be impacted in multiple areas. The main difference between good analytical practices and formal accreditation is the amount of documentation to be developed. There is no doubt that any good analytical laboratory uses qualified analysts, checks the performance of equipment used for testing, and validate methods. However, many times the outcome of the tests is not fully documented. ISO/IEC 17025 accreditation requires formal documented environment – ‘what is not documented is a rumor,’ and is viewed by assessors as ‘not being done.’
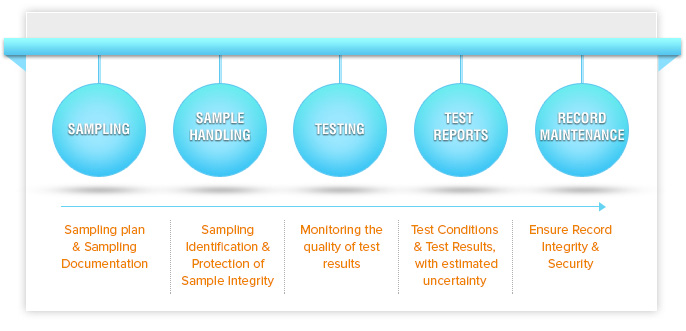
The overall impact of accreditation on a testing laboratory can be best illustrated by looking at the whole sample/data workflow. Figure 1 shows a typical laboratory workflow of samples and test data, together with ISO/IEC 17025 requirements.
COMPLIANCE ACROSS ALL WORKFLOW STEPS
- Validation of analytical methods & Procedures
- Equipment calibration testing & maintenance
- Qualification of material
- Traceablility
- Control of nonconforming testing
- Qualification of personnel
- Controlled environmental conditions
- Written procedures
COMPLIANCE ACROSS THE LABORATORY
Documentation control, corrective & preventive actions, complaint handling, suppler & subcontractor management, non-conflicting organizational structure, internal audits
Requirements Overview
Requirements along the analytical workflow
- Sampling should be performed according to a sampling plan, and all sample details should be documented
- Samples should be uniquely identified and the sample integrity should be protected during transport and storage.
- The quality of test results should be monitored.
- Test reports should include test results as well as an estimation of the overall measurement uncertainty. The report should also include either detailed information about the sample and test conditions, or a link to a reference document.
- Records should be properly maintained to ensure data integrity and availability.
Some requirements impact more than one workflow step:
- All analytical methods and procedures should be validated. This includes methods and procedures for sampling, testing and data evaluation.
- Equipment used for sampling and testing should be calibrated, tested, and well maintained. Material such as calibration standards should be qualified and traceable to System International (SI) units or to certified reference material.
- Nonconforming test results should be documented and controlled.
- People should be qualified for their assigned tasks through education, experience, or training.
- Environmental conditions such as temperature, humidity, and electromagnetic interference should be monitored and controlled.
-
All routine tasks should be performed according to written procedures.
Some additional requirements impact not only sample analysis, but also the organization of the entire laboratory:
- Specific documents should be developed and maintained, including individual policies and a quality plan.
- Known existing problems should be corrected and an action plan should be developed to avoid recurrence of the same or similar problems.
- All complaints from clients should be formally followed up.
- A formal program should be used to manage suppliers, service providers, and subcontractors.
- The organizational structure should be such that there are no conflicting interests that could impact quality.
- Compliance with ISO/IEC 17025 and internal procedures should be assessed during regular internal audits.
Key Steps towards Accreditation:
There are eight key steps towards laboratory accreditation:
- Management defines a project owner
- The project owner studies details of the standard, supporting literature, and other relevant information
- The project owner defines the preliminary scope of accreditation and works with laboratory professionals to prepare a list with requirements
- The project owner and laboratory professionals perform a gap analysis to determine the difference between the requirements and what is currently implemented in the laboratory.
- Based on the outcome of the gap analysis, the project owner, laboratory
professionals, financing and documentation professionals, and external consultants estimate the costs for accreditation - Estimated costs are presented to management, along with incremental opportunities.
- Management decides to proceed with accreditation.
- The project owner leads implementation steps.
Contact us at info@lakshy.com to get your test laboratory ISO 17025 accredited.
Send Enquiry !
Our Services
Our Clientele
The Logos are the property of the respective companies. There are use here just for the representation sake















